

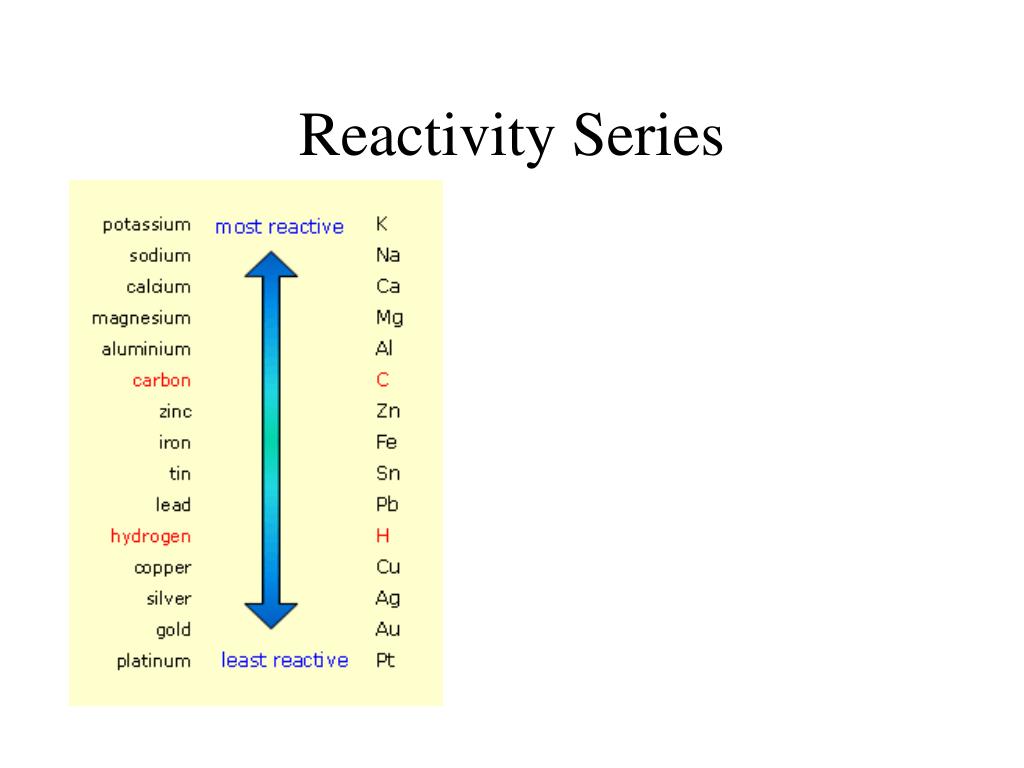
A correlation study between dissolution, cell viability and uptake indicated cell viability and dissolution had a strong negative correlation for Fe 3O 4, and Co xFe 3− xO 4 whereas for Cu xFe 3− xO 4 this correlation was very weak. the more vigorously it reacts with other substances the more easily it loses electrons to form positive ions (cations) We can examine the reactivity of metals by observing their reactions. Calculations show that both Al I and K + work in concert and determines the reactivity of 1. A549 cells showed a dose dependent response (10–200 μg mL −1) and the reduction in cell viability followed the trend of Mn xFe 3− xO 4 > Co xFe 3− xO 4 > Zn xFe 3− xO 4 > Cu xFe 3− xO 4 > Fe 3O 4. DFT calculations confirm that the Al center in 1 is more reactive than that in ( DIPP BDI)Al. If the oxide layer is damaged, the aluminium metal is exposed to attack, even by water. DFT calculations performed on the ferrites to calculate the vacancy formation energy of Fe and dopant atoms substantiated the experimental dissolution data. In accordance with the testing method proposed, the most reactive powder studied was STPA-4 ultrafine aluminum powder produced by electrical explosion of. Aluminum (Al) has been widely used in micro-electromechanical systems (MEMS), polymer bonded explosives (PBXs) and solid propellants. Compared to ferrite nanoparticles, Co and Mn doping significantly enhanced the dissolution, whereas doping with Cu and Zn had an opposite effect to dissolution. The doping % within the ferrites was within the range of 15–18% for all the dopants. Iron and aluminium are extracted from their ores in various ways. The reactivity of a metal is related to its tendency to form positive ions.
#Aluminum reactivity series#
Monodispersed and phase pure variants of ferrites (M xFe 3− xO 4 where M = Co, Cu, Zn, Mn) were synthesised with a size range of 9–11 nm using a wet chemistry route. The reactivity series shows metals in order of reactivity. Part of Combined Science Chemical changes Revise. Iron and aluminium are extracted from their ores in various ways. Stearic acid is an effective lubricant for aluminum because the long-chained acid molecules are chemically attached to the native aluminum oxide surface film. This study looks to investigate as to whether ferrite nanoparticles can be safely and viably doped with transition metal elements without adversely affecting the stability and toxicity of the nanoparticles. The reactivity of a metal is related to its tendency to form positive ions.

Owing to their remarkable properties in terms of electrical resistivity, chemical stability, and saturation magnetisation, ferrite nanoparticles are being increasingly used for a wide range of applications.


 0 kommentar(er)
0 kommentar(er)
